INTRODUCTION
Organic fertilizers such as farmyard, chicken manures and green manures, waste water and natural industrial waste are of high importance due to their supplement to plant nutrient and to sustainable agricultural land by providing soil organic matter, sequent production of food of high quality. However, the direct effect of these wastes may cause new problems droved from the presence of heavy metals, pathogenic organisms, bad odors or phototoxic organic compound (Diez et al. 2001; Garcia et al. 1991). These risks can be minimized by stabilizing the organic matter contained in these wastes through composting (Togneti et al. 2005; Zbytniewski and Buszewski 2005 and Pascual, et al. 1997).
During composting organic compounds are transformed through the successive activities of different microbes to a more stable and complex organic matter. The rate and extent of the starting materials composition are important factors concerning nutrient improved synchronization between nutrient mineralization and nutrient up take. Composting is the decomposition or break down of organic waste materials by mixed population of micro-organisms (microbes) in the warm moist, aerated environment. Non-composting or immature organic composts may pose a number of problems during storages, marketing and use. The negative impact on plant growth is because reduced oxygen or available nitrogen or the presence of phytotoxic compounds (Kabata, et al. 1991, Zheljazkov, 2002).
Furthermore, composting is of great significance from the stand point of weed seeds, plant pathogens, public hygiene, pollution control and environment protection (Lampking, 1990). The use of organic fertilizer will result in a substantial cut in the farmer's fertilizer bill by providing free soil amendment. In addition, collection of waste materials and their conservation keeps villages clean and promote healthy life, reducing trash and retaining soil moisture and consequently saving water bills.
All organic matter eventually decomposes and many methods were developed for compost preparation as traditional or rapid composting practices involving individual or combined application of treatment like shredding and frequent turning, mineral nitrogen compounds, effective microorganism, forced aeration, and mechanical turning. Land traditional methods adopt an approach of aerobic or anaerobic decomposition methods.
Availability of inorganic fertilizers in
A significant shift can be made by using composted natural organic fertilizers e.g. farm yard manure (FYM), chicken manure (CHM) and filter cake (FC), sugar industry organic waste on large scale. Organic manures from different sources are available in large amounts in
Table (1) Preliminary chemical analysis of farmyard manure and chicken manure.
N % P % K % Ca % Mg % Na % Fiber % Cu Mg/kg Fe Mg/kg Mn Mg/kg FYM 1.15 0.40 1 1.75 2.25 0.53 32.0 0.16 15.7 0.865 CHM 1.37 0.82 1.8 2.5 3.00 0.36 14.0 0.28 8.75 1.04
The total weight of the organic pile was 300 kg in a volume of 2m3 (2 x 1 x 1m) and the following additives (treatments) were added to FYM and mixed thoroughly:
1- Control treatment without additives (FYM).
2- Thirty kg of Chicken manure were added to FYM (FYM: CHM 10: 1).
3- Fifteen kg Chicken manure was added to FYM (FYM: CHM 20:1).
The treatments were sampled at 15 day intervals for a period of four months. The treatments were replicated three in a complete randomized design (CRD) and the means were separated using
Analytical Methods:
The concentrations of calcium, magnesium, iron, manganese and copper were determined according to AOAC (1990) using atomic absorption spectrophotometer, model 2380, Perkin Elmer, using air acetylene flame. Sodium and potassium were determined by flame photometer described by Chapman and Pratt (1961) Crude fiber, nitrogen and phosphorus were determined as described by AOAC (1990). The pH was measured using
RESULTS AND DISCUSSIONS
Nitrogen content
Significant differences were observed in the nitrogen content in two weeks sampling in farm yard manure treated with chicken manure (FYM:CHM 10:1) compared to control and farm yard manure treated with chicken manure(FYM:CHM 20:1) (Figure1). The nitrogen contents in the composted material increased and there was a gradual decrease of the bulk volume of the compost. A highly significant increase (P ≤ 0.0001) in nitrogen content was noticed in all weeks in the FYM:CHM (10:1) and the control. The increase in the nitrogen content may be attributed to the release of the organic-bound nitrogen resulted from FYM decomposition and the nitrogen content in chicken manure. Similar results were obtained by Abo Sedera (1995) and Habib et al. (2001) who showed increased nitrogen content with time in composted sugar beets.
Phosphorus content
Phosphorus concentration increased up to the 8th week in treated farm yard manure (FYM: CHM 10:1) as shown in Figure 2. This may be explained by the gradual increase in the microbial activity that may reach its peak after 12th week of biodegradation indicating a positive relation between the microbial biomass and the phosphorus content. It may also be attributed to the rapid decomposition of chicken manure, which enhances the phosphorus release to the soil (Eltilib et al., 1994; Abdelgani, 1997). The phosphorus content of farm yard manure treated with chicken manure decreased significantly up to 12th and 14 th week. The rate of phosphorus mineralization increased as the organic acids built up in the medium due to activates phosphates enzymes, thus, phosphorus was complexes again organically and becomes difficult to be mineralized and released after the 14th week (Elsheikh, 1998). The phosphorus content increased significantly after the 12th week. Perhaps, the level of organic acids decreased and the activity of microorganisms increased again. All treatments showed a general increasing trend of phosphorus content with time. The phosphorus content of FYM:CHM 10:1 treatment and FYM:CHM 20:1 increased compared to control. Similar result was obtained by Elsheikh (1998) who indicated that the nitrogen contained in chicken manure significantly increased the phosphorus content through the enhancement of the microbial activity and hence the organic matter degradation.
Potassium content:
The potassium content of treated farmyard manure was initially less than the control as shown in Figure 3. This could be explained by the release of potassium ions from FYM to the soil as illustrated by Dewes and Schmitt (1994). The potassium content started to increase significantly (P≤ 0.0001) due to composting up to the 14th week in FYM: CHM 10:1 and to the 8th week in FYM: CHM 20:1 treatment, respectively. Chicken manure treatment increased potassium content significantly at the 12th week compared to other treatments by accelerating the biochemical degradation of organic matter and hence the formation of potassium carbonate that conserve potassium from being released to soil solution. Potassium content in chicken manure treated FYM was significantly decreased with time compared to the control. These results agree with the findings of Habib, et al. (2001).
Calcium content
The calcium content was decreased up to the 6th week in treated farmyard manure (Figure 4) The high calcium content in FYM: CHM 10:1 treatment offer an explanation for the high calcium estimated in chicken manure treatment. In addition, the heat energy dissipated from chicken manure decomposition activates the soil microorganisms especially those in charge of phosphates enzyme production that enable phosphorus mineralization and calcium (Abdelgani, 1997; Elsheikh, 1998). Accumulation of organic acids from the decomposition of organic matter results in a drop in the media-pH which enhances the complixation of calcium ions with the organic anions. In the first six weeks calcium content in FYM was increased due to the acidifying effect of organic acids production. After the 6th week, this acidifying effect tended to decrease due to the accumulation of ammonium ions that increased the pH and thus, enhancing more calcium precipitation (Awadalla and Basioni, 1993). After the 12th week, the organic acids accumulated and consequently the pH decreased which favored calcium re-complications. Therefore, the calcium content in the FYM: CHM (10:1) started to increase again significantly compared to the control due to accumulation of organic acids after the 12th week which reduced the pH and thus enhanced calcium complixation.
Magnesium content
Treated farmyard manure showed an increasing trend of magnesium content up to the 9th week (Figure 5). The magnesium content of control was significantly reduced up to the 8th week. This could be due to the low initial magnesium content. Magnesium content increased with time due to the capturing of magnesium ions by organic acids produced by organic matter decomposition (Elsheikh, 1998). Chicken manure treated farm yard manure (FYM:CHM10:1) significantly increased magnesium content compared to other treatments due to acceleration of FYM decomposition and thus, the formation of magnesium organic salts (Awadallah and Basioni, 1993). The depression of Mg content occurred at the 12th week could be explained by the release of magnesium ions from FYM to the soil due to the high activity of soil microorganisms.
Sodium content:
Sodium exhibits a minor importance in plant growth. Generally the sodium content in all treatments was lower than the other cations measured. A significant decrease in the sodium content was noticed between the 4th and the 8th week. The high sodium content in chicken manure treated FYM could be attributed to the originally high sodium content of chicken manure (Figure 6). Sodium content in treated FYM (FYM:CHM 20:1) increased significantly from the 11th week up to the 12th week and dropped in FYM:CHM 20:1 however, the sodium content increased from the10th week up to the 14th week and dropped again. This finding was similar to that obtained by Egrinya et al. (2001) who found increasing sodium content with time due to the decomposition of the organic materials and may be due to the low micro-organisms activity.
Crude fiber content
A highly significant (P≤0.0001) decrease in crude fiber content was observed in all treatments after the 8th week (Figure 7). The first 4 weeks exhibited a significant decrease of crude fiber between treatments relative to the control due to the high microbial activity. As the time goes, a considerable accumulation of microorganisms occurred, and then a gradual decrease in crude fiber content of FYM as the log phase of microbial growth was enhanced. The farmyard manure treated with chicken manure (FYM:CHM 10:1 and 20:1) decreased the crude fiber but the differences were not significant. The decrease may be due to the accumulation of the organic acids that lowered the pH and consequently the microbial activity declines (Elsheikh, 1993). The organic acids tended to dissociate with time and hence the media retain their suitable pH that enhances the microbial activity, which decrease in crude fiber content of FYM as the log phase of microbial growth was enhanced. The farmyard manure treated with chicken manure continued the degradation. Generally the crude fiber has a high decomposition rate compared to cellulose, and considered as a substrate for cellulose enzymes and cellulolytic microorganisms since it contains 95% cellulose.
pH value
The pH value of the compost was initially acidic as shown in Figure 8 but tended to be gradually alkaline due to the release of ammonium (NH4) up to the 6th week and it drops again up to the 10th week. Similar result were obtained by Sara et al. (2003) but this result is in contrast with the results obtained by Habib et al. (2001) who observed a gradual decrease in pH value towards acidity under anaerobic condition compared with the aerobic compost made from sugar beet.
Trace elements
Copper content
Significant differences were observed in the copper content in all treatments (Figure 9). Highly significant increase (P ≤ 0.0001) in copper content in the 2nd week in farmyard manure treated with chicken manure (FYM: CHM 10:1). The farm yard manure treated with chicken manure (FYM: CHM 10:1) increased the copper content relative to the control and FYM: CHM 20:1 in the 10th week.
Iron content
Iron concentration increased up to the 8th week in treated farmyard manure (FYM: CHM 10:1) and (FYM: CHM 20:1) as shown in Figure 10. In the last week (16th), the iron content of FYM:CHM 10:1 treatment significantly increased (P≤0.0001) compared to the control and FYM: CHM 20:1 treatments.
Manganese content
The manganese content started to increase significantly (P≤ 0.0001) due to composting up to the 8th week in all treatments (Figure 11). The manganese content increased significantly, but in the 10th week, the manganese content of chicken manure treated farm yard manure (20:1) was high compared to other treatments. Similar result was obtained by Egrinya et al. (2001) who found that the compost was high in iron and low in copper content, they also found highly significant difference (P≤0.0001) in manganese. Various factors such as pH, original organic material and kind of microorganism might have influenced the decomposition of organic material thus, increasing release of trace elements. Vallcho et al. (2004) noticed increasing trace elements content with time due to decomposition of organic material.
Micro organism identified
The micro-organisms identified at the three stages of temperature are listed in Table 3 namely bacteria isolates at mesophilic, thermophilic and cooling down stage of composting. The bacteria isolates at mesophilic, thermophilic and cooling down stage indicated that there was great diversity. The bacteria isolation obtained at misophilic stage is shown in Table 3; however bacillus spp. isolated at the mesophilic stage were again found at the thermophilic stage and they also persisted during the cooling down stage. Similar results were obtained by the Taiwo and Oso (2004). They found that other micro-organisms not found at this elevated temperature might have been adversely affected by the thermal effect of the heat generated by microbial activities. They also observed that the microbial activities were directly related to the availability of energy sources and inorganic nutrients required for their growth.
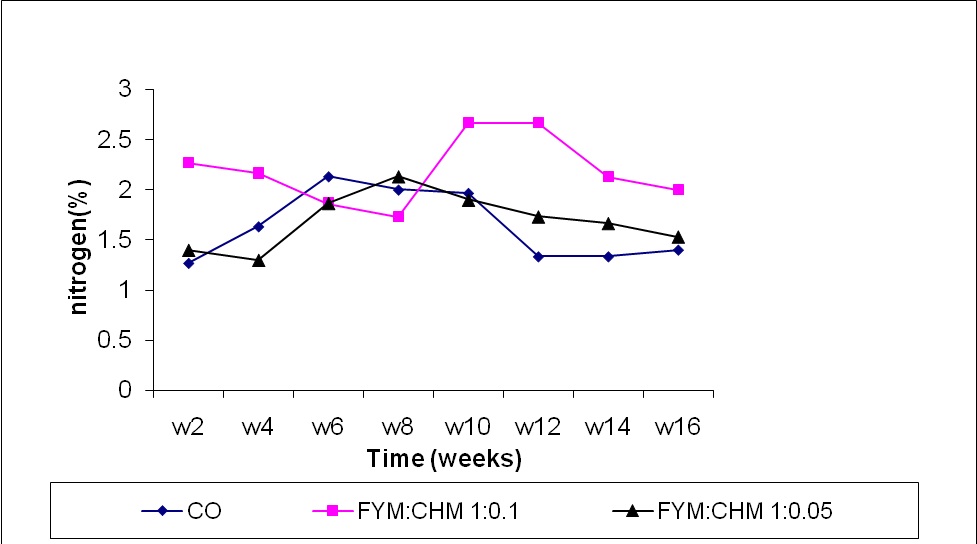
Figure (1) Effect of treatments and time on nitrogen content.
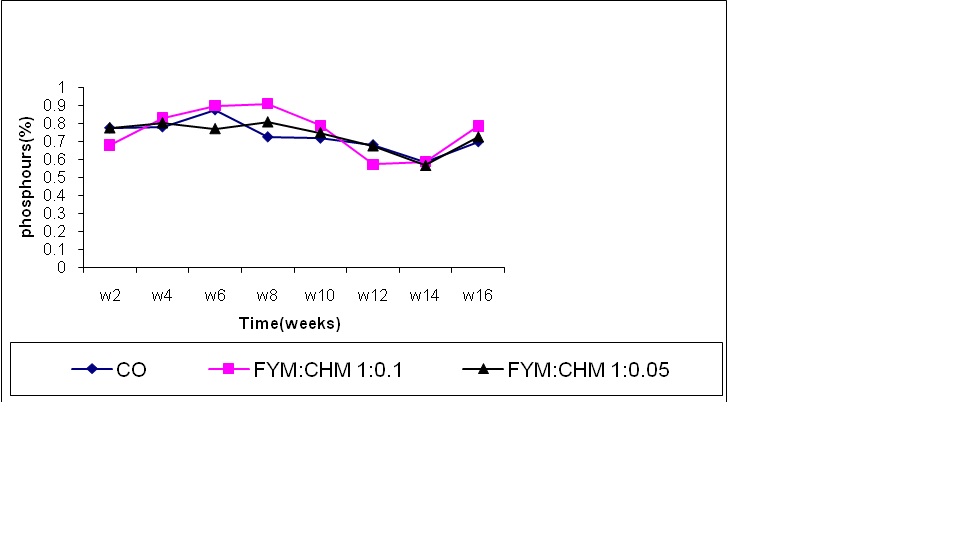 |
Figure (2) Effect of treatments and time on phosphorus content.
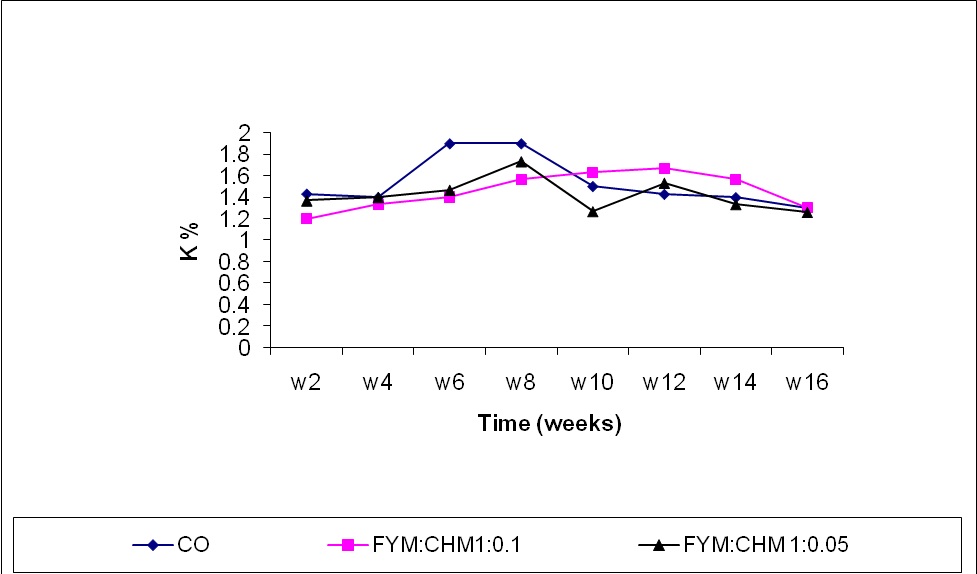 |
Figure (3) Effect of treatments and time on potassium content
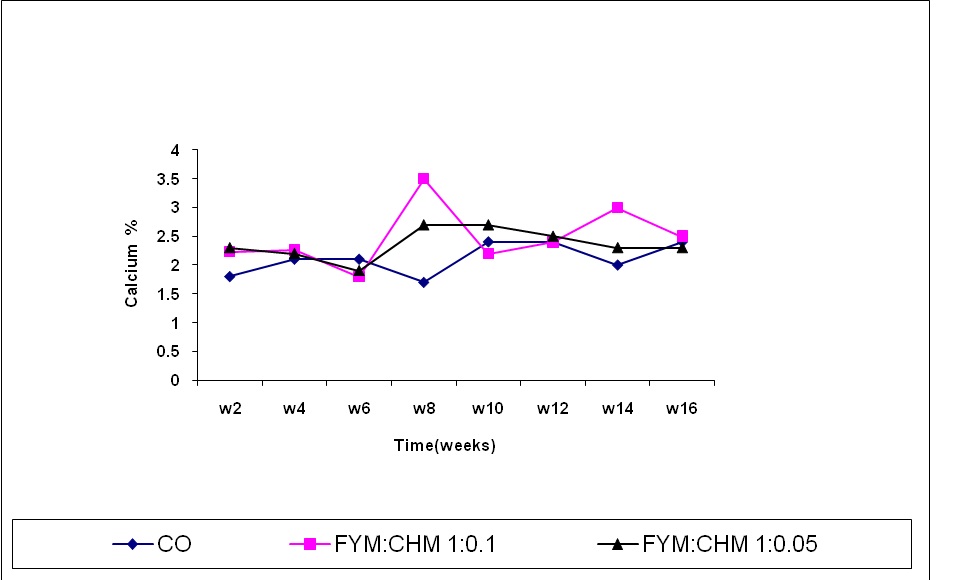 |
Figure (4) Effect of treatments and time on calcium content
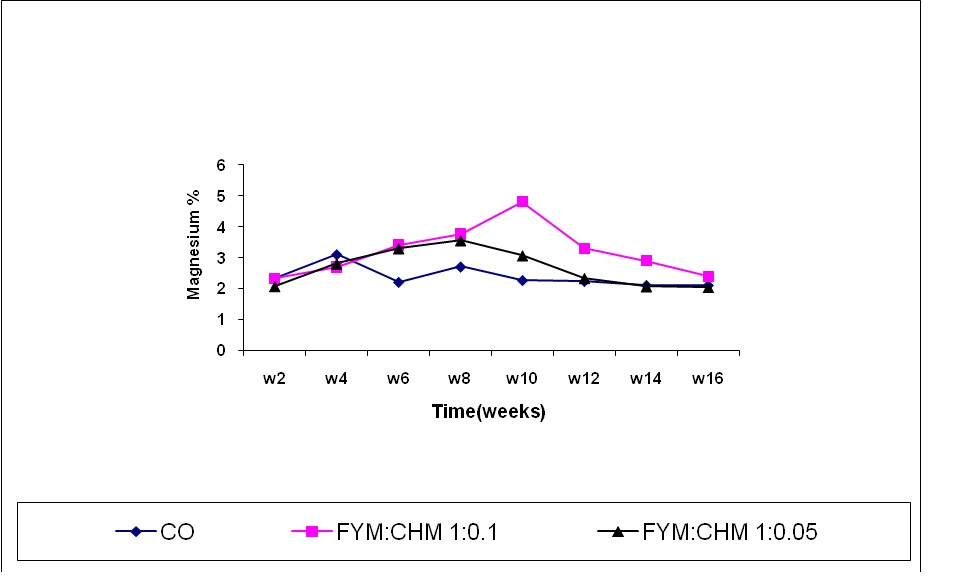 |
Figure (5) Effect of treatments and time on magnesium content.
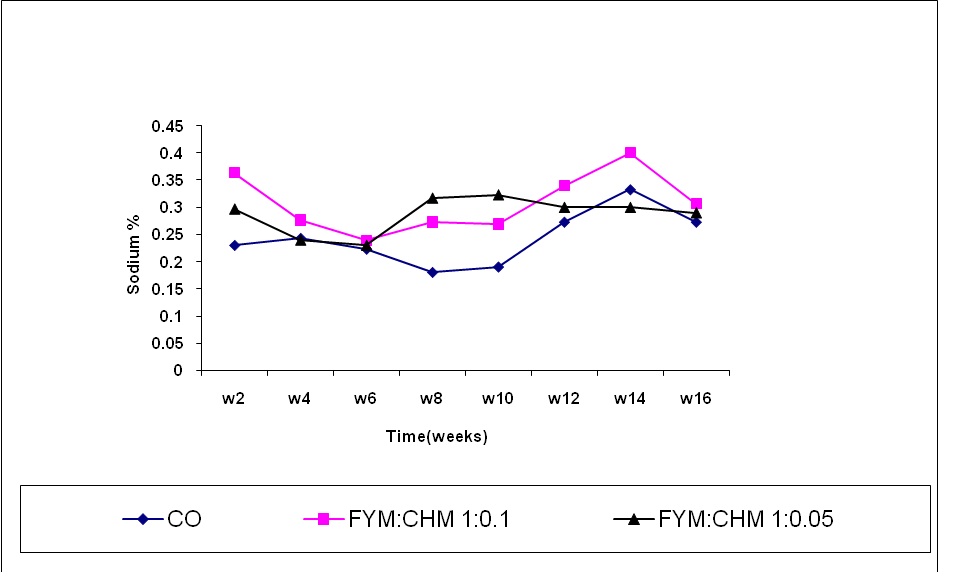 |
Figure (6) Effect of treatments and time on sodium content.
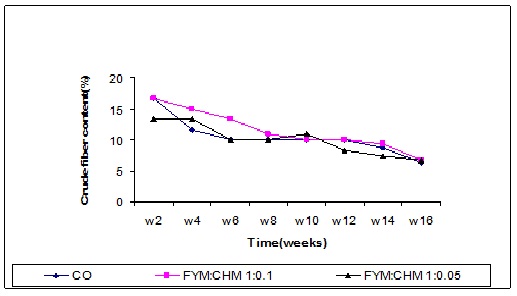
Figure (7) Crude fiber content as affected by duration of decomposition.
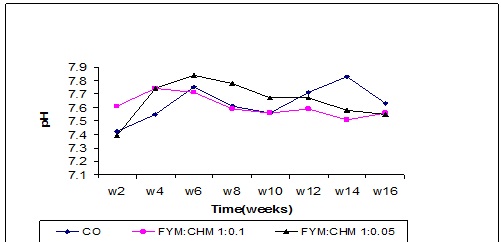 |
Figure (8)Effect of treatments and time on pH
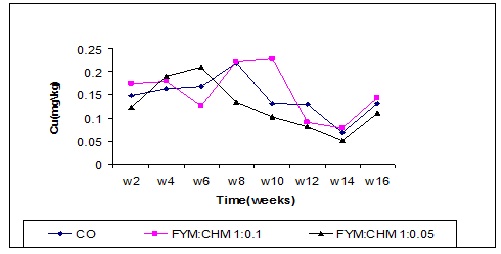 |
Figure (9) Effect of treatments and time on copper content
 |
Figure (10) Effect of treatments and time on iron content
Figure (11) Effect of treatments and time on manganese content
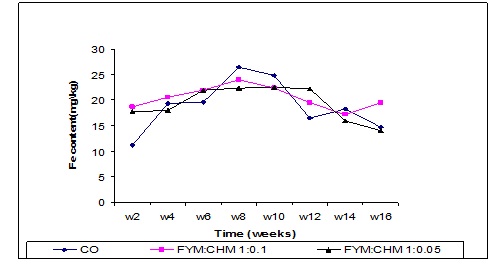 |
Table 2: Total viable count of bacteria (CFU\ml)
|
|
Table 3: Bacteria isolation at mesophilic, thermophilic, and cooling down stages
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
P = present
A = absent
REFERENCES
Abdelgani, M. E. (1997). Effect of Rhizobium on nitrogen fixation, yield and seed quality of Feanugrek (Trigonella foenum graecum L), pH. D (Agric) Thesis, University of Khartoum,Sudan
Abo-Sedera, S. A. (1995). Biological and chemical studies on organic wastes decomposition PH.D Thesis Fac- (Agric) AL-AZHAR University - Egypt.
AOAC (1990). Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed. Suite 400, 2200 Wilson Boulevard, Arlington, Virginia, USA.
Awadalla E.A. and Basioni N. H. (1993). Fertilizers and fertilization (in Arabic) Istealition compu.Center Gyro University pp140
Brenner, D. J., (1984). Facultative Anaerobic gram negative Rods .Sec.5.In: Bergey's Manual of systematic Bacteriology, (Ed.) and vol (1):409-598.
Dewes, T. D. and L. S. Schmitt. (1994). Deposition of nitrogen and potassium from farmyard manure heaps in the soil under long-term manure storage areas. Agrobiological Research 47:115-120.
Diez, J. A.; DeIaTorre, A. I.; Cartagena M.C and Vallejo. A. ; Munoz, M. J. (2001) Evaluation of application of pig slurry to an experimental crop using agronomic and erotological approaches J. Environ Qual. (30):2165-2172 R
Elsheikh, E. A. (1993). Soil microbiology (in Arabic) K. U.:108-218.
Elsheikh ,A.E and Ekhlas,M.m. (1998).Effect of biological organic and chemical fertilizer on yield ,hydration coefficient, cook ability and mineral composition of groundnut seed .Food Chemistry, (36) No 2 pp:253-257.
Eltilib, A. M.; Ali, A. M. and Abdullah, M. A. (1994). Effect of Chicken manure and salinity on growth and leaf .nitrogen ,Phosphorus and potassium content on Okra growth in two soil types K.U.J Agric.Sci , I(2): 16-36.
Epstein, E. (1997). Trace elements heavy metals and micronutrients in the science of composting Technomic, puble, AGL Ancaster P.A, pp 137-170.
Eringanejie (2001). Physicochemical changes in livestock feces during composting. Soil, Sci. Plant .Analysis P: 477-489.
Garcia; C.; Hernandez,T.;Costa,F.Ayuso,M. (1991) Evaluation of the maturity of municipal waste composts using simple Chemical parameters Comm. Soil SCI plant Annual (23):1501-1512
Habib, F.M; Negm, M.A. and M.M.Hassan (2001). Composting of Sugar Beet Residues. A study on condition and period of composting Egypt. J. Agric. Res, 79(2) PP: 373-383.
Harrigan, W.F., and Maccance, M. E. (1976). Laboratory methods in microbiology.P:257-303Academic Press. London and New York.
Hoitink, H. A. J. (2000). Trends interments and utilization of solid wastes through composting in United States in proceeding of the international compost symposium. Warman, P.R; Toylor, B.R,.E as ,G,B,A press Truro NS Canada pp:1-13
Kabata, A.; Pendias. H. (1991). Trace elements in soil and plant .2n Ed; Kabata Pendices, A. H. Eds, CRC Press Boca Ratan FLP: 365.
Lampking, N. (1990). Organic Farming .Farming press pp: 86-102.
MekkiI. I. (1997) Comparative study on the fertilizing effect of biogas sludge on yield of wheat and on soil microbial population PH.D (Agric) Thesis, University of Khartoum .Sudan.
Pascual, J. A. A.; Yuso, M.; Garcea, C.; Hernadez, T. (1997). Characterization of urban wastes according to fertility and phytotoxic parameters, waste mange Res (15):103 -112.
Sara, A. M., Nour, A., Samy, A. M. (2003). Production of organic compost from organic residual. Natural resource and environment annual report, pp, 107-112.
Schnitzer, M. and Khan (1987). Humic Substances in the Environment. Marcel, Pekker, publ NY.
Sneh Gogal, S. K.; Dhull, K. K. Kapoor. (2005). chemical and biological changes during composting of different organic wastes and assessment of compost maturity bio resource technology (96):1584-1591.
Sulieman, M. H. (1996). Preparation of organic fertilizers. M.Sc. (Agric) Thesis University of Khartoum, Sudan.
Taiwo, L.B., and Oso, B.A. (2004). Influence of composting techniques on microbial succession, temperature and pH in a composting municipal solid waste. African Journal of Biotechnology vol 3 (4). pp: 239-243.
Tognetti, C.; Laos, F.; Mazzarion, M. J.; Herndez, M. T. (2005). Composting us .verm-composting a comparison of end product quality compost Sci. Util (13):6-13.
Valtcho, D., Zheljazokov and Philip, Warman, R. (2004). Application of high Cu compost to Dill and peppermint. J. (Agric) food Chem,52, pp2615-2622.
Zheljazkov, V. D.; Warman, P. R. (2002). Bio availability of Cu in compost amended soil in The Proceedings of the International Compost Symposium; Warman P. R.; Taylor. B. R.; CBA press Truro NS Canada (2):697- 715.
Zytniewski, R. and Buszewski, B. (2005). Characterization of natural organic matter (Nom) derived from sewage sludge compost-part1 chemical and spectroscopic properties Bioresearch Technology, 96:471-478.
جديد الاقسام
- Potential of Renewable Energy in Sudan
- تداعيات وآفاق اقتصادات الصناعة البستانية فى السودان
- الأنتاج ومخاطر الاستثمار البستاني فى السودان
- الأنتاج وفرص الاستثمار البستاني فى السودان
- تداعيات وآفاق اقتصادات الصناعة البستانية فى السودان
- مدني 27-6-2020 (سونا)- أعلنت إدارة مشروع الجزيرة عن إنطلاق زراعة محاصيل العروة الصيفية للموسم الزرعي (2020-2021)م .
- مجلة جامعة الجزيرة للعلوم الزراعية
- مجلة جامعة الخرطوم للعلوم الزراعية
- مكتبة الموسوعة السودانية
- المكتبة
- وزارة الزراعة والغابات يلتقي بوفد مزارعي ولاية كسلا
- القريب فروت
- ملتقى تفاكري حول الكيماويات الزراعية (حالة المبيدات ) يوم الاثنين
- ملتقى تفاكري حول الكيماويات الزراعية (حالة المبيدات ) يوم الاثنين
- وزارة الزراعة بالخرطوم تؤكد على ضرورة وضع سياسات و اضحة لتقنين الأراضى الزراعية
- بدء الاحتفال بيوم الزراعة العربي بمقر المنظمة العربية للتنمية الزراعية بالخرطوم
- وعاء الزروع يحقق أعلي نسبة تحصيل لجباية الزكاة للنصف الأول من العام الجاري
- السفير التركي لدى السودان يعلن استعداد تركيا لانفاذ مشاريع زراعية كبرى بولاية نهر النيل
- المنظمة العربية للتنمية الزراعية تؤكد اهتمام الدول العربية بالمخزون الاستراتيجي للسلع الغذائية
- وزير الزراعة يؤكد أهلية السودان لتحقيق الأمن الغذائي العربي







